crosslinking theory of aging
Glycation And Aging
The crosslinking theory of aging was postulated in 1942. Intra or intermolecular crosslinking of bio-macromolecules (protein, DNA and lipids) contributes to the cellular senescence and physiology of aging. Glycation and Methylation are 2 crosslinking mechanisms of aging attracted the most attention and studies. We will discuss glycation in this section and methylation in the next section.
Glycation (sometimes called non-enzymatic glycosylation) refers to a type of chemical reaction in which glycating agents such as sugar molecule fructose or glucose, form covalent bond to a protein or lipid molecule without the controlling action of an enzyme. All blood sugars are reducing molecules. Glycation may occur either inside the body (endogenous glycation) or outside the body (exogenous glycation). Enzyme-controlled addition of sugars to protein or lipid molecules is termed glycosylation; glycation is a haphazard process that impairs the functioning of biomolecules, whereas glycosylation occurs at defined sites on the target molecule and is required in order for the molecule to function.
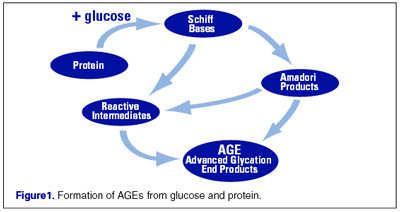
Endogenous glycations occur mainly in the bloodstream to a small proportion of the absorbed simple sugars: glucose, fructose, and galactose. The balance of the sugar molecules is used for metabolic processes. It appears that fructose and galactose have approximately ten times the glycation activity of glucose, the primary body fuel. Glycation is the first step in the chains of a complex series of very slow reactions in the body known as Amadori reactions, Schiff base reactions, and Maillard reactions; all leading to the formation of advanced glycation end products (AGEs) in that the glycated protein cross link to other functional protein molecule to form the AGE. The intermediate products are known as Amadori, Schiff base and Maillard products. Side products generated in intermediate steps may be oxidizing agents (e.g. hydrogen peroxide), or not (e.g. beta amyloid proteins). Examples of AGE modified sites in proteins are carboxymethyllysine (CML), carboxyethyllysine (CEL) and Argpyrimidine which is the most common epitope.
Exogenous glycations (dietary or pre-formed) and advanced glycation end products (AGEs) are typically formed when sugars are cooked with proteins or fats. Temperatures over 120°C (~248°F) greatly accelerate the reactions, but lower temperatures with longer cooking times also promote their formation. Browning reactions (usually Maillard type reactions) are evidence of pre-formed glycations. Indeed, sugar is often added to products such as French fries and baked goods to enhance browning. Food manufacturers have added AGEs to foods, especially in the last 50 years, as flavor enhancers and colorants to improve appearance. Foods with significant browning, caramelization, or with directly added preformed AGEs can be exceptionally high. A very partial listing of foods with very high exogenous AGEs includes: donuts, barbecued meats, cake, and dark colored soda pop. These compounds are absorbed by the body during digestion with about 30% efficiency.
Glycation (cross-linking) of (structural) proteins, lipids and DNA disrupts the functions of these molecules, interfere with molecular and cellular functioning throughout the body. Body levels of AGEs increase with age. Although some AGEs are benign, but others are more reactive than the sugars they are derived from. Crosslinked proteins accumulate with time and eventually disrupt cellular function and is associated with a number of age-related phenotype and diseases. The mechanism of glycation and AGE accumulation in accelerating aging have 3 aspects: crosslinking itself, free radical intermediate byproducts such as hydrogen peroxide and other harmful intermediate side products, and the associated increase in chronic inflammation. The presence of AGEs in a glycated tissue can result in a multifold increase in the rate of production of free radicals compared to that in an unglycated tissue.
In addition, Many cells in the body (for example endothelial cells, smooth muscle or cells of the immune system) from tissue such as lung, liver, kidney or peripheral blood bear the Receptor for Advanced Glycation End products (RAGE) that, when binding AGEs, result in the production of cytokine chemicals that can induce unwanted and potentially deadly inflammation in blood vessels, nerve, liver and other tissues. The result of AGEs can be self-propagating systemic tissue inflammation. These age-related chronic inflammatory diseases include atherosclerosis, asthma, arthritis, myocardial infarction, nephropathy, retinopathy or neuropathy.
Glycated substances are eliminated from the body slowly, since the renal clearance factor is only about 30%. This implies that the half-life of a glycation within the body is about double the average cell life. Long-lived cells (such as nerves, brain cells), long-lasting proteins (such as eye crystalline and collagen), and DNA may accumulate substantial damage over time. Metabolically-active cells such as the glomeruli in the kidneys, retina cells in the eyes, and beta cells (insulin-producing) in the pancreas are also at high risk of damage. The table below summarize the glycation, AGE induced pathophysiology and chronic diseases.
| molecular causes | tissue damaged | type of disease | disease |
| crosslinked collagen | skin | skin aging | wrinkle |
| crosslinked collagen | blood vessel walls | high blood pressure, micro- or macro-aneurisms | |
| crosslinked collagen | brain | stroke | |
| beta cell damage in pancreas (produce insulin) | type I and II diabetes | ||
| artery wall (endothelium) | cardiovascular disease | atherosclerosis | |
| myelin | age-related neurological disorders | peripheral neuropathy | |
| amyloid proteins (intermediate side products) | age-related neurological disorders | Alzheimer’s disease | |
| acrylamide and other side-products | cancer | ||
| kidney | nephropathy | ||
| demyelination | sensory organ damage | deafness | |
| microvascular damage in the retina | sensory organ damage | blindness | |
| crosslinked crystalline | lens of eye | cataract |
Collagen,the most common protein molecule in our bodies, forms the connective tissue that provides structure and support for organs and joints. When glucose binds with collagen—as it tends to do as we age—this normally supple protein loses much of its flexibility. As a result, lungs, arteries, tendons, and other tissues stiffen and become less efficient.
The above aging pathophysiology appear at younger ages in people with diabetes, who have high glucose levels (hyperglycemia). Glycated hemoglobin in red blood cells is an important marker physicians use to measure hyperglycemia. While the physiological effects of glycated hemoglobin are unclear, the disease it helps doctors to detect—diabetes—is sometimes considered an accelerated model of aging. Not only do the complications of diabetes mimic the physiologic changes that can accompany old age, but people with this condition have shorter-than-average life expectancies. AGE formation can be increased beyond normal levels in diabetic patients. As a result, much research on crosslinking has focused on its relationship to diabetes as well as aging. Red blood cells have a consistent lifespan of 120 days and are easily accessible for measurement of glycated hemoglobin level known as HbA1c in order to monitor blood sugar control in diabetes.
Hyperglycemia results in higher cellular glucose levels in those cells unable to reduce glucose intake (e.g. endothelial cells). This in turn results in increased levels of NADH and FADH,(generated in glucose metabolic pathways) increasing the proton gradient beyond a particular threshold at which the complex III (one of the enzyme complex of mitochontria electron transport chain as discussed before in free radical theory of aging) prevents further increase by stopping the electron transport chain. This results in mitochondrial production of reactive oxygen species (ROS) byproduct much more than the normal level, activating PARP1 (Poly (ADP-ribose) polymerase family, member 1 is a human gene which encodes a chromatin-associated enzyme, poly-ADP ribose polymerase-1). Poly-ADP ribose polymerase-1 in turn, ADP-ribosylates GAPDH, a protein involved in glucose metabolism, leading to its inactivation and an accumulation of metabolites earlier in the metabolism pathway. These metabolites activate multiple pathogenic mechanisms, one of which includes increased production of AGEs. AGE are believed to play a causative role in the vascular complications of diabetes mellitus eg. diabetic retinopathy and diabetic neuropathy.
As has been mentioned in “Introduction”, our body has an internal “maintenance, repair and defense mechanism/system” that would repair and protect us from the various damaging effects. Usually this repair and defense system functions well while we are young, however when we progressively become more and more aged, this system itself also deteriorates and declines together with other body functions. The intra or inter-molecule crosslinkings (glycation, methylation and other types of crosslinking) are no exception. Body’s internal degradation and excretion of AGE, cells of immune system (macrophage) and Carnosine, the natural anti-glycation dipeptide work together to combat the deleterious effects of glycation and AGE crosslinking.
Our body has an internal system to degrade (by protease) and excrete AGE (by kidney) although glycated substances are eliminated from the body slowly. Cellular proteolysis of AGEs produces AGE peptides and “AGE free adducts” (AGE adducts bound to single amino acids) which, after being released into the plasma, can be excreted in the urine. The resistance of extracellular matrix proteins to proteolysis, renders AGEs of these proteins less conducive to elimination. Larger, extracellularly-derived, AGE proteins cannot pass through the basement membrane of the renal corpuscle and must first be degraded into AGE-peptides and AGE free adducts. Peripheral macrophage as well as liver sinusoidal endothelial cells and Kuppfer’s cells have been implicated in this process although the real-life involvement of the liver has been disputed
While the AGE free adducts are released directly into the urine, AGE-peptides have been shown to be endocytosed by the epithelial cells of the proximal tubule and subsequently degraded by the endolysosomal system to produce AGE-amino acids (i.e. AGE free adducts). The AGE-amino acids are hypothesized to then be exported back into the lumen of the nephron for subsequent excretion. AGE free adducts are the major form through which AGEs are excreted in urine with AGE-peptides occurring to a lesser extent but accumulate in the plasma patients with chronic renal failure. With advancing age, both protease and our kidney become less efficient or dysfunctional, the ability in removal of accumulated AGE declines contributing to the steady increase of AGE levels.
Immune cells called macrophages can combat glycation. Macrophages with special receptors for AGEs seek out and engulf them. Once AGEs are broken down, they are ejected into the blood stream where they are filtered out by the kidneys and eliminated in urine. Levels of AGEs increase steadily with age. One reason is that kidney function tends to decline with advancing age. Another is that macrophages, like certain other components of the immune system, become less active as we age.
Carnosine is a multifunctional dipeptide made up of amino acids beta-alanine and L-histidine that exists both in food and in human body. It is a unique dipeptide that interferes with the glycation process, preventing cross-linking of proteins. Carnosine occurs in very low concentrations in the brain and other tissues (muscle). Long-lived cells such as nerve cells (neurons) and muscle cells (myocytes) contain relatively higher levels of carnosine compared to cells from other tissues because long-lived cells accumulates substantially high level of AGE. Carnosine levels decline with age. Muscle levels decline 63% from age 10 to age 70, which may account for the age-related decline in muscle mass and function. In the laboratory, carnosine has been shown to delay the senescence of human fibroblasts.
Because AGEs are related to elevated blood sugar levels, a low calorie diet (Calorie Restriction) may reduce AGE body load. Carnosine has been used as an anti-aging supplement. Visit our “anti-aging treatment” knowledge base to find more information about Carnosine. AGEs are the subject of ongoing research. AGE crosslink breaking drugs are currently being developed for the purpose of breaking crosslinks between proteins. Alt-711, also called alagebrium developed by Synvista Therapeutics is the first of these to reach clinical trials. Glycation inhibitors include benfotiamine, pyridoxamine, taurine, aminoguanidine, carnosine, and aspirin. It is also believed that alpha-lipoic acid and acetyl-L-carnitine can also reduce glycation damage.