A consensus panel convened by the American Association of Cancer Research has defined a CSC as “a cell within a tumor that possesses the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor.” These tumor-forming cells could hypothetically originate from stem, progenitor, or differentiated cells. As such, the terms “tumor-initiating cell” or “cancer-initiating cell” are sometimes used instead of “cancer stem cell” to avoid confusion. The CSC hypothesis therefore does not imply that cancer is always caused by stem cells. Rather, tumor-initiating cells possess stem-like characteristics to a degree sufficient to warrant the comparison with stem cells; the observed experimental and clinical behaviors of metastatic cancer cells are highly reminiscent of the classical properties of stem cells.
The CSC hypothesis suggests that the malignancies associated with cancer originate from a small population of stem-like, tumor-initiating cells. In 1994, Lapidot and colleagues provided the first solid evidence to support the CSC hypothesis when they used cell-surface protein markers to identify a relatively rare population of stemlike cells in acute myeloid leukemia (AML). Subsequent analysis of populations of leukemia-initiating cells from various AML subtypes indicated that the cells were relatively immature in terms of differentiation. In other words, the cells were “stem-like” more closely related to primitive blood-forming (hematopoietic) stem cells than to more mature, committed blood cells.
The identification of leukemia-inducing cells has fostered an intense effort to isolate and characterize CSCs in solid tumors. Stem cell-like populations have since been characterized using cell-surface protein markers in tumors of the breast, colon, brain, pancreas, and prostate. In other cancers in which CSCs have yet to be identified, researchers are beginning to link established stem-cell markers with malignant cancer cells. For instance, the proteins Nanog, nucleostemin, and musashi1, which are highly expressed in embryonic stem cells and are critical to maintaining those cells’ pluripotency, are also highly expressed in malignant cervical epithelial cells. While this finding does not indicate the existence of cervical cancer CSCs, it suggests that these proteins may play roles in cervical carcinogenesis and progression.
Do CSCs Arise From Stem Cells
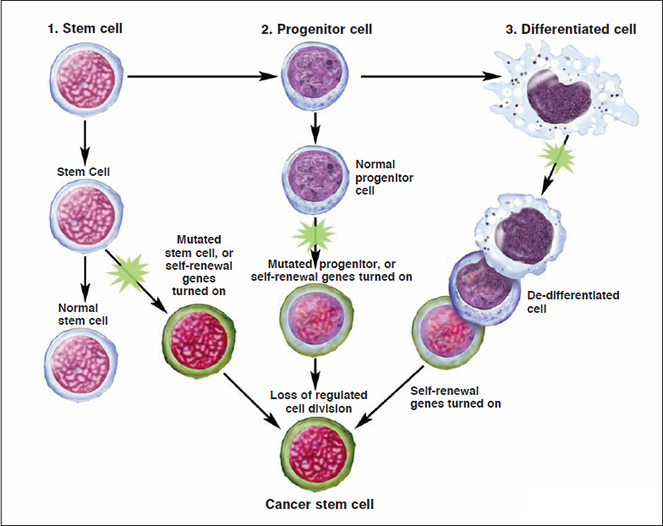
Given the similarities between tumor-initiating cells and stem cells, researchers have sought to determine whether CSCs arise from stem cells, progenitor cells, or differentiated cells present in adult tissue. this section will review several theories about the cellular precursors of cancer cells.
If CSCs arise from normal stem cells present in the adult tissue, de-differentiation would not be necessary for tumor formation. In this scenario, cancer cells could simply utilize the existing stem-cell regulatory pathways to promote their self-renewal. The ability to self-renew gives stem cells long lifespans relative to those of mature, differentiated cells. It has therefore been hypothesized that the limited lifespan of a mature cell makes it less likely to live long enough to undergo the multiple mutations necessary for tumor formation and metastasis. Several characteristics of the leukemia-initiating cells support the stem-cell origin hypothesis.
The differentiation pathway from a stem cell to a differentiated cell usually involves one or more intermediate cell types. These intermediate cells, which are more abundant in adult tissue than are stem cells, are called progenitor or precursor cells. They are partly differentiated cells present in fetal and adult tissues that usually divide to produce mature cells. However, they retain a partial capacity for self-renewal. This property, when considered with their abundance relative to stem cells in adult tissue, has led some researchers to postulate that progenitor cells could be a source of CSCs.
Some researchers have suggested that cancer cells could arise from mature, differentiated cells that somehow de-differentiate to become more stem celllike. In this scenario, the requisite oncogenic (cancer causing) genetic mutations would need to drive the de-differentiation process as well as the subsequent self-renewal of the proliferating cells. This model leaves open the possibility that a relatively large population of cells in the tissue could have tumorigenic potential; a small subset of these would actually initiate the tumor. If a tissue contains a sufficient population of differentiated cells, the laws of probability indicate that a small portion of them could, in principle, undergo the sequence of events necessary for de-differentiation. Moreover, this sequence may contain surprisingly few steps; researchers have recently demonstrated that human adult somatic cells can be genetically “re-programmed” into pluripotent human stem cells by applying only four stem-cell factors.
How Cancer Stem Cells Could Support Metastasis
Metastasis is a complex, multi-step process that involves a specific sequence of events; namely, cancer cells must escape from the original tumor, migrate through the blood or lymph to a new site, adhere to the new site, move from the circulation into the local tissue, form micrometastases, develop a blood supply, and grow to form macroscopic and clinically relevant metastases.
Some researchers have proposed that these unique cells may be CSCs. In this hypothesis, metastatic inefficiency may reflect the relative rarity of CSCs combined with the varying compatibilities of these cells with destination microenvironments. Researchers have demonstrated that stem cells and metastatic cancer cells share several properties that are essential to the metastatic process, including the requirement of a specific microenvironment (or “niche”) to support growth and provide protection, the use of specific cellular pathways for migration, enhanced resistance to cell death, and an increased capacity for drug resistance. There is emerging, albeit limited, evidence that these properties may also hold for CSCs. Moreover, normal stem cells tend to be quiescent unless activated to divide. If the CSC hypothesis holds true, then undifferentiated, dormant CSCs would be relatively resistant to chemotherapeutic agents, which act mainly on dividing cells. As such, this subpopulation could form the kernel of cells responsible for metastasis and cancer recurrence following treatment and remission.
How The CSC Model Could Affect Cancer Therapy
As noted previously, most contemporary cancer treatments have limited selectivity systemic therapies and surgeries remove or damage normal tissue in addition to tumor tissue. These methods must therefore be employed judiciously to limit adverse effects associated with treatment. Moreover, these approaches are often only temporarily effective; cancers that appear to be successfully eliminated immediately following treatment may recur at a later time and often do so at a new site. Agents that target molecules implicated in cancer pathways have illustrated the power of a selective approach, and many researchers and drug developers are shifting toward this paradigm. If the CSC hypothesis proves to be correct, then a strategy designed to target CSCs selectively could potentially stop the “seeds” of the tumor before they have a chance to germinate and spread.
There is also some evidence to suggest that CSCs may be able to selectively resist many current cancer therapies, although this property has yet to be proven in the clinic. Normal stem cells and metastatic cancer cells over-express several common, known drug-resistance genes. Experiments in cell lines from breast cancer and glioma have shown that CSCs (as identified by cell-surface markers) are more resistant to radiotherapy than their non-CSC counterparts. In the face of radiation, the CSCs appear to survive preferentially, repair their damaged DNA more efficiently, and begin the process of self-renewal. These discoveries have led researchers to propose several avenues for treating cancer by targeting molecules involved in CSC renewal and proliferation pathways. Potential strategies include interfering with molecular pathways that increase drug resistance, targeting proteins that may sensitize CSCs to radiation, or restraining the CSCs’ self-renewal capacity by modifying their cell differentiation capabilities. In each case, successful development of a therapy would require additional basic and clinical research.